In-Vivo Phototherapy: Shining a Light on the Flexible Photonic iCarP Patch
Technical Analysis | 03-07-2023 | By Liam Critchley
Imagine a world where the treatment of deep tissue diseases is not only more effective but also safer and more versatile. This is the promise of phototherapy, a medical technique that uses light to treat a variety of conditions. But how do we overcome the challenges of delivering light energy to deep tissues? The answer might lie in a new device called iCarP.
What if we could treat deep tissue diseases more effectively, safely, and versatilely? This is not a distant dream but a reality brought closer by a new device called iCarP.
The Promise and Challenges of Phototherapy
Phototherapy is a medical technique that allows for the illumination of organs and tissues and can be used in a range of clinical settings, including in optical diagnostic tests, laser surgery, for treating the skin of newborn babies, and for any other procedure where light-activated therapies create a unique light microenvironment on a target of interest.
Phototherapies are used within a wide range of clinical settings and are a common technique in hospitals. Despite their widespread use, and like many things that are used today, there is always some room for improvement.
Overcoming Obstacles: The Role of In-Vivo Illumination Strategies
One of the key areas that has the potential to be improved is the precision, versatility, and controllability in delivering the light energy to the target. In a lot of phototherapy procedures, the in-vivo illumination strategies used don’t have a depth, area, wavelength, and power to be used universally across different clinical areas.
In simpler terms, 'in-vivo illumination strategies' refer to the methods used to deliver light energy directly to the target area within the body. These strategies need to be versatile and adaptable, as the depth, area, wavelength, and power required can vary greatly depending on the specific clinical application.
This is especially true when trying to create a large area illumination into deep tissue targets, so if these factors can be controlled to a greater degree, then it could improve the safety and efficacy of phototherapy procedures, as well as widen the application scope in where they are used.
When it comes to trying to deliver light energy to deep tissue and organ targets, it is hard for light to penetrate so far into the body because the light particles are absorbed by the surrounding tissues, the photons get scattered by biological media, and the autofluorescence of nearby organs and organelles can interfere with the procedure.
Current Implantable Light Sources for In-vivo Phototherapy
There have been a number of studies that have come out with a range of implantable light-emitting components. Common examples include near-field-communication (NFC)-based light-emitting-diodes and upconversion nanoparticles. In these areas, the energy-transferring efficiency or the light penetration efficiency of the device determines its illumination intensity.
Optical fibre-based devices are another option for more precise and high-power waveguiding operations and can be made nowadays from clinically approved biocompatible materials. Fibre optics are also more versatile than other devices, not only because they are a very established technology in the communication space, but they can also be integrated easily with endoscopes and used with magnetic resonance imaging (MRI).
There’s an interest in fibre-optic-based devices because they can be placed within a target location of the body to illuminate a specific area. This means that they can be used in a number of clinical areas, including laser-induced thermotherapy, optogenetics, and temperature and pressure sensing. Fibre optic devices can also be integrated within tissues for deeper illumination targeting.
The New iCarP Light Emission Platform
Researchers have now created a flexible and biodegradable photonic device, known as iCarP, that can illuminate internal tissues and organs within the human body. The device is composed of a refractive polyester patch, a removable tapered optical fibre, and a micrometre scale air gap between these two components. The iCarP uses the diffraction of the optical fibre, with dual refractions in the air gap, and reflections inside the patch to achieve a bulb-like illumination that can be guided towards the target organ or tissue. Unlike many other fibre optic photonic devices, the iCARP devices are placed parallel to the target tissue of interest rather than being embedded within it.
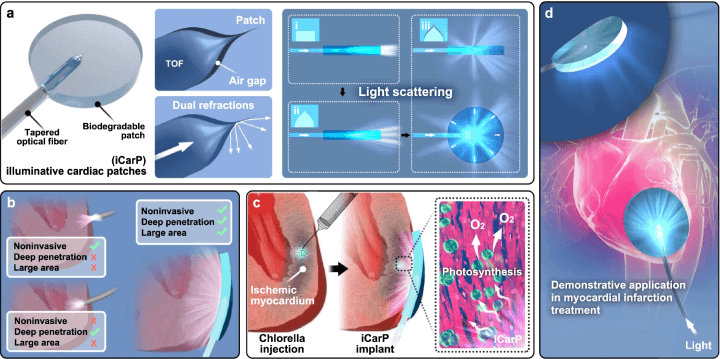
a) The iCarP's structure comprises a tapered optical fiber (TOF) and a waveguiding patch substrate, separated by an air gap. This design enhances light scattering, particularly lateral illumination, compared to configurations without an air gap. b) iCarP's illumination effects outperform those of an optical fiber alone, providing noninvasive, deep, and large-area illumination. c-d) When applied to intramyocardially injected chlorella, iCarP's illumination triggers in situ photosynthesis within the infarcted myocardium, demonstrating its potential in treating myocardial infarction. (Click to enlarge)
The illumination properties of the iCarP device can cover a large area, are of high intensity, can be used continuously or in pulse mode, and can deeply penetrate the target tissue without puncturing it. The illumination from the device has also shown that it can support many different photosensitisers that are used throughout different phototherapy procedures.
For instance, consider a patient undergoing phototherapy for deep tissue cancer. With traditional methods, the light energy might not reach the target area effectively, reducing the efficacy of the treatment. However, with the iCarP device, the light can penetrate deeply and cover a large area, potentially improving the treatment outcome.
While light scattering is a key design feature for achieving illumination, the patch also protects the fragile fibre optic tip from the mechanical stresses caused by the local tissue and protects the device from contamination. The optical fibre used transmits both visible and infrared light, so the device can be used to deliver light with a low tissue penetration so that it doesn’t damage the internal tissue. Likewise, there is no circuitry within the iCarP device, so there is no Joule heat generated by the device, making the risk of thermal injury almost non-existent. Because the patch is biodegradable and the optical fibre is removable, no toxic or non-degradable residues are left behind after treatment.
Future Potential of the Platform
The research done in this study has shown that the iCarP is a safe and powerful device for illuminating internal organs and tissues in phototherapy-based treatments and diagnostic approaches. The study has already shown that the photonic device is compatible for thoracoscopy-based minimally invasive implantation onto beating hearts, but there are other areas where the device could be explored for future use.
The researchers conducted a series of in-vivo experiments to test the effectiveness of the iCarP device. They found that the device was able to deliver high-intensity illumination to deep tissue targets without causing any damage to the surrounding tissues. Furthermore, the device was found to be biodegradable, leaving no toxic or non-degradable residues after treatment. These results demonstrate the potential of the iCarP device for use in a wide range of phototherapy applications.
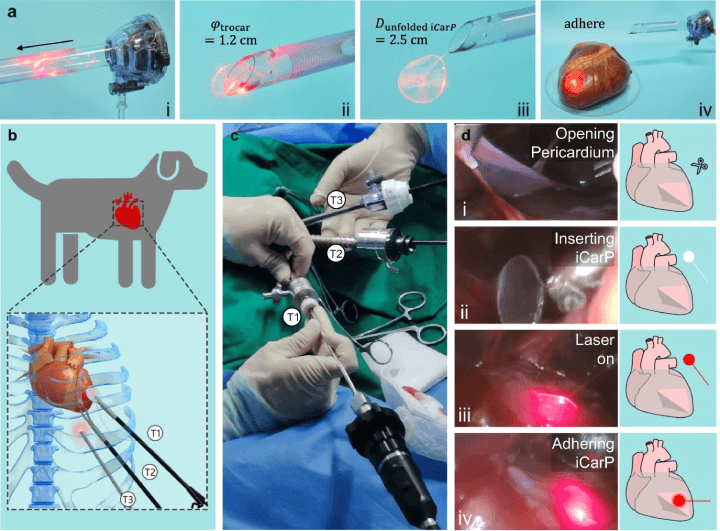
The iCARP device's passage through a trocar is as follows: (a) iCarP, initially folded to fit a 1.2 cm diameter trocar, is inserted (i), passes through the exit (ii), and unfolds once it has passed through (iii). The unfolded iCarP is then adhered to the surface of a porcine heart (iv). The minimally invasive implantation of iCARP in a canine model is illustrated in (b), with trocar 1 (T1) for grasping forceps, trocar 2 (T2) for curved scissors, and trocar 3 (T3) for an endoscope. The iCARP implantation in dogs is performed under thoracoscopy without open-heart surgery (c). The implantation steps include opening the pericardium (d-i), inserting the iCARP (d-ii), turning on the illumination (d-iii), and adhering the iCARP (d-iv). (Click to enlarge)
Because the device can illuminate repeatedly over a long timeframe, the device could be trialled in future research in optogenetics, photo-sensitive drug release and targeting, and light-based sensing/diagnosis applications. It’s also thought that photonic devices could be used to not only deliver optical energy but also be a carrier of information as well. This could be exploited for integrating medical data communication into biomedical applications and could be used to integrate therapy and diagnosis in a single closed-loop device.
Conclusion:
The advent of the iCarP device marks a pivotal moment in the realm of phototherapy. This innovative tool not only promises to enhance the effectiveness of treatments for deep tissue diseases but also paves the way for safer and more versatile therapeutic approaches. As we delve deeper into the capabilities of iCarP, we find ourselves on the precipice of a transformative era in phototherapy. The potential applications of this technology extend beyond our current understanding, promising to redefine the boundaries of healthcare. The journey of discovery is just beginning, and the future of phototherapy is bright, quite literally. As we continue to unlock the potential of devices like iCarP, we eagerly anticipate the transformative impact these advancements will have on the future of healthcare. The path forward is illuminated with promise, and only time will reveal the full extent of this revolution in phototherapy.
Reference:
Zhu Y. et al., A biodegradable, flexible photonic patch for in vivo phototherapy, Nature Communications, 14, (2023), 3069: https://www.nature.com/articles/s41467-023-38554-x

