Electrolyzers: Mass Hydrogen Extraction from Water
Technical Analysis | 07-09-2023 | By Gary Elinoff
Through the process of electrolysis, powered by wind or solar energy, we can produce an abundant supply of energy-rich green hydrogen. This innovative technology not only captures the immense potential of renewable sources but also transforms them into a sustainable fuel. As we grapple with the challenges of climate change and energy security, the role of electrolysis, facilitated by electrolyzers, becomes pivotal in shaping a cleaner and more sustainable energy landscape.

The Role of Electrolysis in Green Hydrogen Production
Electrolysis, a standout among green technology examples, involves a critical process where electricity is used to initiate a reaction that splits water molecules into hydrogen and oxygen. This principle underlies the operation of commercial electrolyzers. Conceptually, the process is quite simple, as illustrated by the diagram below.
According to the US Office of Energy Efficiency and Renewable Energy, there are various methods of electrolysis, each utilizing different types of electrolyzers. Each type has its advantages and challenges, but all contribute to the production of green hydrogen when powered by renewable energy sources.
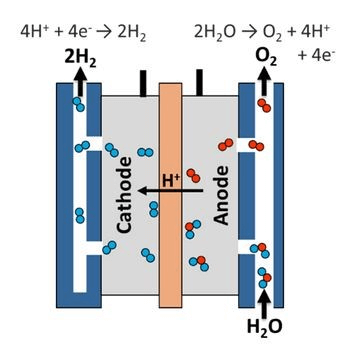
Generalized electolyzer. Image source: The US Office of Energy Efficiency and Renewable Energy1
Electricity is supplied across the anode and cathode, and water enters at the anode. The space between the anode and cathode, bisected by a permeable membrane (illustrated as a brown strip in the diagram), is filled with an electrolyte. The electricity at the anode breaks every two water molecules down to one oxygen molecule, four hydrogen ions (H+) and four electrons (e-). The hydrogen ions are attracted to the negatively charged cathode and will transverse the membrane to get there. It is here that the four hydrogen ions combine with the four electrons to produce two hydrogen molecules.
Green Hydrogen
Green hydrogen, typically produced through the electrolysis process, ensures no environmentally damaging byproducts are generated, such as carbon dioxide (CO2), and if our electrolyzer is powered by green electricity, such as wind or solar, it certainly fits the bill.
We’ll get back to why green hydrogen is so important in so many ways later on, but first, we’ll discuss the electrolyzers themselves and who makes them. A list of the top 20 manufacturers are listed here.
Alkaline Electrolyzers
Alkaline electrolyzers are a well-established and widespread technology. An alkaline solution is basic (pH greater than 7) rather than acidic, and these type of electrolyzers derive their name from the alkaline nature of the electrolytes they employ, typically potassium hydroxide (KOH) or sodium hydroxide (NaOH).
As mentioned in Science Direct, alkaline electrolyzers have been in use for over a century and have a proven track record of safety and reliability. They are particularly suitable for large-scale hydrogen production due to their robustness and scalability.
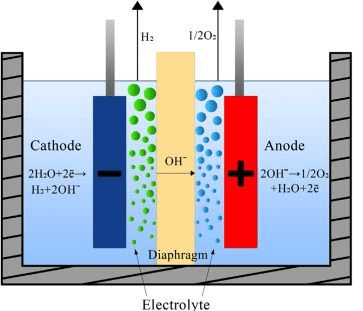
Alkaline electrolyzer. Image source: Science Direct2
The reaction at cathode is: 2 H2О + 2e− → H2 + 2 OH−
While at the anode: 2 OH− → 1/2 O2 + H2O + 2e−
With a bit of algebra, the overall reaction can be demonstrated to be: 2 H2O →2 H2 + O2
There is also a second type of alkaline electrolyzer. In this variation, designated the zero gap design, the cathode and anode both touch upon the separator membrane.
Alkaline electrolyzers are relatively inefficient as compared to the newer technologies now available. However, they are a stable, extremely mature modality, and they are inexpensive.
Polymer Electrolyte Membrane (PEM) Electrolyzer
The Cummins HyLYZER3, which can convert 20 megawatts of electricity into H2 gas, is said to be the largest PEM electrolyzer in the world. Polymer electrolyte membrane electrolyzers are also known as proton exchange membrane electrolyzers. They differ from alkaline electrolyzers largely in the electrolytes they employ. The PEM electrolyzers employ an ionically conductive solid polymer electrolyte, as opposed to the liquid electrolytes employed by alkaline electrolyzers. Another difference is that for PEM devices, it is H+ ions rather than OH- ions that diffuse across the membrane.
Additionally, for a given amount of electrical input, PEM electrolyzers produce more hydrogen gas than alkaline units. However, the PEM units require higher operating pressure than the alkaline devices. While alkaline electrolyzers have been employed for over a century, the PEM electrolyzer was developed in the 1950s and 1960s for the space program, and it was the O2, not the H2, that was the sought-after derivative.
The Cummins HyLYZER, commissioned in January 2021, stands as a testament to the advancements in PEM technology. Located in Bécancour, Quebec, it showcases the potential of PEM electrolyzers in large-scale hydrogen production.
Solid Oxide Electrolyzer (SOE)
The SOE is the third major type of electrolyzer. This type of unit employs solid ceramic materials as the electrolyte. The operation of the device involves bathing the cathode with steam, which, combined with electrons from the electricity source, produces negatively charged oxygen ions along with the desired hydrogen gas. To complete the circuit, the oxygen ions must pass through the electrolyte, reaching the anode and to emit oxygen.
While PEM and alkaline units operate at temperatures of approximately 80℃ and 100℃, respectively, SOE’s must function at around 750℃. However, because SOE units take advantage of the waste heat produced by other chemical applications, overall efficiency is improved. SOE’s are a relatively new technology.
Storing and Transporting Hydrogen Gas
The greatest problem with the use of hydrogen is storing and transporting it. At room temperature, hydrogen gas isn’t very energy-rich. But, like most gasses, it can be compressed for correspondingly higher energy density.
The problem here is that hydrogen atoms are the smallest atoms in the universe. Typically, hydrogen is stored in vessels or pipes made of far larger atoms, such as steel. The lattice structures of iron are huge compared to the size of hydrogen, and the gas, especially under pressure, can easily worm their way into the empty spaces between adjacent iron atoms. The result is that transport pipes of storage vessels can be quickly degraded.
Freezing Hydrogen Gas into a Solid
One of the benefits here is that hydrogen’s energy density is at its greatest as a solid. One problem is that to solidify hydrogen gas, it must be reduced in temperature to -259℃, which is within shouting distance of absolute zero, or -273.15℃. This takes a lot of energy, reducing overall efficiency. Worse still, some of the hydrogen will escape its container, further reducing efficiency and creating a hazardous situation for operating technicians.
Politics and Economics
As described in an earlier article, “The Economics of Green Energy: A Case Study of Wind Ranchers in Texas” 4, science and technology aren’t the only problems associated with green energy. In a video entitled “World’s Dirtiest Clean Energy Project (and Why I’m a Fan)” 5, a hydrogen collaboration between Australia and Japan is described.
Both countries collaborated on a vessel capable of transporting solid hydrogen from Australia across the Pacific Ocean to Japan. Specialized ports were built to accommodate the ship at the Australian source and to unload it in Japan.
The interesting thing is that electrolyzers were not employed. Rather, Australian hydrocarbon, too dirty and of too low an energy content to be viable for sale under ordinary conditions, was employed to generate the hydrogen.
There are two takeaways here. One is that a method of freezing, loading and transporting solid hydrogen across a vast ocean was demonstrated. The other is that the project would likely have never happened without the support of the owners of the hydrocarbons. Additionally, there is the expectation that local labour in Australia would be employed as the project expands.
The video "World's Dirtiest Clean Energy Project (and Why I'm a Fan)" by Engineering with Rosie provides a visual representation of the challenges and opportunities in the hydrogen energy sector. It's a must-watch for anyone interested in the future of green energy.
The obvious next step is to dump the low-grade hydrocarbon and instead employ green energy and electrolyzers to produce the hydrogen.
The Economics of Green Energy article on Electropages highlights the financial feasibility of green energy projects. With the right investments and collaborations, green hydrogen can become a cost-effective and sustainable energy source for the future.
Why Electrolyzers are so Vital for a Green Future
There are innumerable uses for hydrogen in agriculture and industry. As an example, hydrogen is a vital input to the Haber Process, which produces the ammonia (NH3) necessary for the synthesis of the agricultural fertilizers upon which the lives of billions of people depend.
At present, most commercial H2 is generated by an extremely dirty process known as steam methane reforming. It uses vast amounts of energy and releases copious amounts of carbon dioxide and even more damaging carbon monoxide into the atmosphere.
Through the use of green energy and the electrolysis process facilitated by electrolyzers, we can meet the world’s growing demand for hydrogen with minimal environmental impact.
References:
- Hydrogen Production: Electrolysis - The US Office of Energy Efficiency and Renewable Energy
- Fuel Cells and Hydrogen Technology – Science Direct
- The HyLYZER – Cummins Corporation
- The Economics of Green Energy: A Case Study of Wind Ranchers in Texas - Electropages
- World's Dirtiest Clean Energy Project (and Why I'm a Fan) – Engineering with Rosie
Glossary:
- Electrolyzer
- A device that splits water into hydrogen and oxygen using electricity.
- Green Hydrogen
- Hydrogen produced without generating environmentally harmful byproducts.
- Alkaline Electrolyzers
- Electrolyzers that use an alkaline solution as the electrolyte.
- PEM Electrolyzer
- Also known as proton exchange membrane electrolyzers, they use a solid polymer as the electrolyte.
- SOE
- Stands for Solid Oxide Electrolyzer, which uses solid ceramic materials as the electrolyte.

