Ingestible Electronics: Non-Invasive Gut Stimulation Technology
Technical Analysis | 02-10-2024 | By Liam Critchley

Key Thing to Know:
- Ingestible electronics offer a non-invasive way to diagnose and treat a variety of GI conditions, with the potential to replace more invasive surgical procedures.
- The IngRI device uses advanced wireless power transfer and hydrogel adhesion technologies to deliver long-term therapeutic electrostimulation within the GI tract.
- Testing on in-vivo pig models has demonstrated that the device can increase ghrelin levels, showing potential for regulating appetite and treating metabolic disorders.
- Future iterations could include integrated sensors and adaptive features for personalised medical treatments, providing real-time feedback and tailored therapies.
Many types of electronics are available nowadays, and a particularly interesting area that is being developed on the interface of technology and healthcare is the field of ingestible electronics. Ingestible electronics have the potential to diagnose and treat a range of different conditions once they have been ingested by a patient.
The gastrointestinal tract (GI) is similar to the brain, heart, and vagus nerve in that it consists of cells that can be electrically excited and modulated by electrostimulation. The GI tract is an important part of the body where the neural, endocrine, and immune systems intersect, and electrostimulation of the GI tract has already been proven in a number of therapies to treat nausea, vomiting, obesity, diabetes and gastric dysmotility. The GI is, therefore, a prime target for electrostimulation from within using ingestible devices.
Why Ingestible Electronics?
Ingestible electronics are appealing for electronically stimulating the GI tract because they are one of the few internal organs that can be accessed via the oral route—unlike the brain, heart and vagus nerve, which all require invasive surgical procedures. Electrostimulation devices that target the GI tract can, therefore, be designed to support self-administration without the need for a medical professional to be present. However, as it stands, ingestible devices for electrostimulation (IDEs) are not yet as effective as their surgical counterparts due to issues around contact, navigation, retention, and powering (C-N-R-P).
C-N-R-P Challenges of Ingestible Electronics
C-N-R-P is preventing ingestible electronics from becoming the preferred method over surgery. In time, this should change as the potential benefits of ingestible electronics far outweigh surgery, but only if these challenges are tackled—and each aspect has different issues that need to be addressed.
Regarding contact, many IDEs experience poor electrode-tissue contact. This arises from random gastric positioning after the IDE has been ingested, alongside the mechanical mismatch of the IDE and the surrounding organic tissue—as many IDEs tend to be rigid with a much different Young's modulus compared to soft gastric tissue. This causes variable and non-therapeutic interactions between the IDE and gastric wall.
For navigation, the majority of IDEs don't possess an autonomous locomotive ability. This prevents the device from precisely positioning inside gastric lumen without endoscopic interventions. This issue affects the effectiveness of the electrostimulation, and in turn, the effectiveness of the therapy.
Retention is also a big issue because most IDEs cannot stay in the gut for more than several hours—which is much lower than surgical gastric stimulators that can remain attached for years. Finally, many of the batteries used in IDEs have a finite life driven by a low power density and are non-rechargeable in nature. Batteries also need to be carefully designed if they're ingested as they are exposed to a highly corrosive gastric environment.
Given the number of challenges with current IDEs, thin and flexible skin-like devices could be the answer because the skin and gastric mucosa share many similarities.
New Device Aims to Overcome These Challenges
Researchers have now created a soft robotic interface called IngRI that is ingestible, battery-free, wireless and can provide acute electrostimulation to the GI tract. The IngRI has been designed to adhere to biological tissue without the mechanical mismatch and has tried to address all of the C-N-R-P challenges that have plagued ingestible electronics to date.
The innovative approach of the IngRI to overcoming C-N-R-P challenges is bolstered by the use of hydrogel adhesion technologies, which draw inspiration from biological processes. Research demonstrates that bio-inspired designs, such as using lyophilized hydrogel, can create a negative pressure when contacting wet surfaces, allowing for secure and prolonged adhesion within the GI tract. This significantly improves tissue contact stability without causing damage to surrounding tissues.
The IngRI has achieved this through a softer material design. The IngRI has been fabricated by creating photolithographic thin-film circuits embedded within a sub-mm thick elastomer substrate. The device was encapsulated in a commercial gelatine capsule so that it could be delivered orally. This soft design enabled the IngRI to conform to the gastric mucosa with a robust and efficient electrode-tissue contact.
Once released from the capsule, an on-board permanent magnet enabled the device to orientate and navigate itself to a specific gastric position using an external handheld magnet. Long-term retention in the gastric mucosa was obtained by activating a hybrid hydrogel/elastomer adhesive interface that enabled the device to stay in place for over 48 hours—enabling electrostimulation to be provided over multiple days.
A programmable electrical pulse was delivered to the GI tract from the device using near-field inductive coupling operated at 13.56 MHz. This frequency was enough to power and modulate the IngRI to deliver therapeutic-level electrostimulation. Power to the device was delivered wirelessly from a transceiver coil to a receiver copper coil in the device, with the coil being wire bonded to a pair of stimulation electrodes for delivering the electrostimulation.
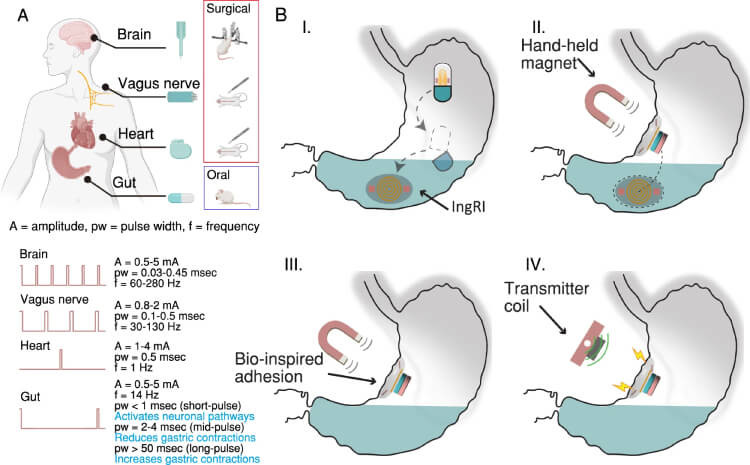
A Therapeutic electrostimulation for tissues and organs that exhibit electrical excitability, alongside the common delivery methods used for such treatments. B The operational process of the IngRI: (I) The gelatin capsule dissolves, releasing the folded IngRI into the gut. (II) The IngRI navigates to the targeted gastric location with the assistance of an external handheld magnet. (III) The IngRI adheres to the gastric mucosa using bio-inspired hydrogel adhesion, enabling effective electrode-tissue contact. (IV) The IngRI administers programmable electrical pulses through near-field inductive coupling. Figure (A) was created with BioRender.com and is licensed under a Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International license.
Advancements in Wireless Power Transfer for Ingestible Devices
Recent advancements in wireless power transfer, such as near-field inductive coupling, are critical in providing the device with enough energy to operate effectively inside the human body. Unlike battery-powered systems, which suffer from limited lifespans and safety concerns, the IngRI utilises external power sources, mitigating the risks associated with battery degradation and exposure to corrosive environments within the gut.
The device was also tested on in-vivo pig models, where the IngRI wirelessly delivered a therapeutic-level electrostimulation through a 10 cm thick abdominal space. The short pulse electrostimulation resulted in an increase in the plasma ghrelin levels, i.e. the 'hunger' hormone that activates the growth hormone secretagogue receptor (GHS-R) to stimulate food intake and fat deposition. This electrostimulation approach could, therefore, be used to regulate appetite and treat other endocrine conditions.
Ghrelin modulation through electrostimulation not only demonstrates the immediate physiological impact of the device but also opens up potential applications in treating metabolic conditions such as obesity and diabetes. By adjusting the frequency and duration of electrostimulation, researchers believe it may be possible to regulate appetite and metabolic functions, offering a non-invasive alternative to current treatment methods.
It's thought that the device could serve as a versatile platform for treating a range of GI conditions where the therapies target the GI mucosal membrane.
Potential for Personalised Medicine and Therapeutic Platforms
Ingestible devices like the IngRI are pushing the boundaries of personalised medicine, enabling the development of platforms that could combine diagnostic and therapeutic functionalities. Future iterations could include integrated sensors that dynamically adjust treatment protocols in response to real-time data, ensuring tailored therapeutic interventions that enhance patient outcomes.
It could be possible to switch stimulation electrodes for vibrational, optical, or thermal sources that could enhance drug absorption through the mucosa membrane. Miniature sensors could also be integrated into the platform to enable closed-loop and on-demand stimulation based on real-time physiological feedback—which could help the micromanagement of dietary cycles in obese patients.
The current iteration of the IDE has addressed some of the key challenges facing the field today and provided prolonged electrostimulation over multiple days. While this is not yet in a realm to challenge the status quo of surgical devices, the hope is that future iteration of the device could have usable lifetimes on the order of weeks or even months. Given that they are simple to administer in out-of-clinic settings, taking a capsule every few months could become a viable alternative to invasive surgical procedures.
In testing, these advanced adhesion techniques were shown to provide superior retention times compared to conventional ingestible devices, maintaining contact with gastric tissues for up to 48 hours in porcine models. This increased retention ensures that therapeutic electrostimulation is delivered consistently over time, maximising the effectiveness of the treatment.
Reference:

