Wearable Interfaces for Gene Therapies: Medical Delivery Solutions
14-08-2023 | By Liam Critchley
As technology advances, the integration of electronic devices with biological systems is becoming a frontier in medical innovation. Wearable devices that can monitor, sense, and even administer therapies are no longer a distant dream but a tangible reality. This article explores the fascinating world of electrogenetic interfaces, a cutting-edge technology that bridges the gap between the digital and biological realms, with a focus on its potential applications in healthcare, including insulin delivery and personalised medicine.

Smart and interconnected wearable devices are becoming more and more popular in our daily lives, especially in the healthcare sector. A lot of wearable devices have centred around monitoring and sensing devices, even though there is the potential for a wide range of therapeutic options. The challenge with many wearable devices is that they don’t have a communication interface that is functional on both the biological and electronic side to administer therapeutic loads effectively.
This is because biological and electronic systems work in vastly different ways. Biological systems are analogue in nature, are programmed by genetics, and are controlled by the flow of ions through protein membranes. On the other hand, electronic systems are digital in nature, are programmed by software and are controlled using a flow of electrons. This often makes the two systems incompatible from an operational perspective, but there is a way to connect the two, and this is through an electrogenetic interface.
In the realm of wearables that can administer drugs, electrogenetic interfaces are the missing link to program and administer gene-based therapies—which could lead to completely compatible systems that operate in both electronic and genetic worlds.
The Biological Aspects to Consider
To develop an effective interface between the biological and man-made worlds, the operations in the biological world must be considered the most. Synthetic biology has evolved over the years to the point where analogue gene switches have been assembled to create complex genetic circuits that can program cellular behaviour.
While all-natural, these systems possess a logic process functionality that is similar to many synthetic, man-made electronic components. Gene circuit ‘components’ assembled in this way have shown to be the natural version of oscillators, timers, memory systems, relay filters and even analogue-to-digital style converters.
These genetic circuits often come into play when medical conditions are being controlled, and many gene switches can be triggered in the presence of small molecular compounds such as antibiotics, vitamins, food additives, cosmetics, or volatile fragrances. They can also be triggered by a number of external stimuli such as light, magnetic field, radio waves or heat, but typically require a high energy input.
An Electrogenetic Interface for Delivering Insulin and Other Therapies
Challenges and Solutions in Electrogenetic Interfaces
The creation of an effective electrogenetic interface that can bring together genetic and electronic systems requires a specific power source. The power source needs to be battery-powered while being able to fine-tune mammalian gene expression over time using a voltage and can connect medical interventions to the Internet of Things (IoT) or Internet of Bodies (IoB).
There have been many solutions attempted that have been successfully implemented in cell cultures but are incompatible in clinical applications due to high levels of cytotoxicity or poor bioavailability. Some have also required high alternating currents, but these need to be controlled with complex bioelectronic implants. A lot of these challenges have made them unsuitable for battery-powered wearable systems, but researchers have now turned to DC-powered electrodes as a solution.
Recent advancements in bioelectronic interfaces, such as the versatile bioelectronic interface (VIBE), offer promising solutions for real-time profiling of bio-analytes and hormone sensing2. This technology could pave the way for individually optimised healthcare through interconnected miniaturised devices2.
Development and Application of the DART System
Electrodes can deliver DC at a low voltage, so much so that it can generate free electrons and radical species, which can lead to the production of radical oxygen species (ROS) at low, non-cytotoxic levels. ROS are commonly used in the body to kill harmful cancer cells and are produced via electron-transfer reactions during respiratory processes and immune responses.
Recent studies have shown promise for DC-based systems. Using DC-based generation of hydrogen peroxide, researchers have previously shown that they can produce an electrogenetic system by using engineered bacterial cellson the surface of the electrode that will initiate a gene expression under electrical stimulation.
Technologies like VIBE are shaping the future of electrogenetic interfaces, allowing for ultrasensitive detection and control of receptor-ligand interactions. Such interfaces could revolutionise the way wearable devices interact with biological systems, opening new avenues for therapeutic interventions2.
Using the DC electrodes and ROS generation capability, researchers aimed to tackle the missing link in electrogenic interfaces1 by creating a device known as DC-actuated regulation technology (DART) that provides DC-powered electrogenetic target gene modulation in human cells1. The DART system enables electrode-mediated, time, and voltage-dependent gene expression in human cells using DC from batteries.
Fig. 1: Schematic of the Mammalian Cell Transgene Expression Switch Activated by Direct Current
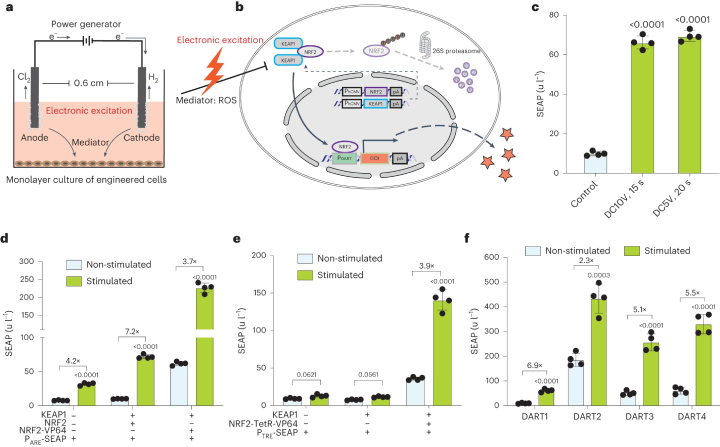
a. A schematic illustration shows the stimulation setup for monolayer cultures. Two platinum wires in each well of a 24-well plate function as anode and cathode, placed 0.6 cm apart in the culture medium. Electric current application results in bubble formation and gas production at the electrodes. b. The electrogenetic circuit is represented schematically, focusing on the NRF2/KEAP1 antioxidative response. Electrical stimulation triggers ROS formation, sensed by NRF2 and KEAP1 complexes, leading to NRF2's nuclear translocation and gene activation. Without stimulation, NRF2 is targeted for degradation. c. SEAP production by transiently transfected HEK293 cells is shown under different DC stimulations. d. SEAP production variations are shown for cells transfected with different combinations of ARE reporter, KEAP1, and NRF2 variants. e. SEAP production is illustrated for cells co-transfected with specific constructs, including NRF2 fused to a tetracycline-dependent transactivator, and stimulated with DC5V for 20 seconds. f. SEAP production by cells co-transfected with different numbers of ARE repeats in the promoter region is shown, with stimulation at DC5V for 20 seconds. (Click to enlarge)
The DART system's groundbreaking approach is supported by recent research, which highlights the potential of electrogenetic interfaces in mammalian gene expression1. This technology could revolutionise the way wearable devices interact with biological systems, opening new avenues for therapeutic interventions2.
The interface consists of genetic components that allow human cells to be responsive to DC-triggered electrostimulation while also enabling DC-adjustable gene expression1. The human cells used were human embryonic kidney cells (HEK293).
To facilitate gene expression, the cells were initially exposed to a 4.5 V DC current for 10 seconds. However, it was found that the level of ROS produced during this initial process was not substantial to undergo the expression. To overcome this, these cells were hypersensitised, which elevated the number of ROS produced and coordinated a genetic response. The DART uses the DC supply to generate the ROS, which then acts as a biosensor to fine-tune the gene expression towards specific genes.1
The researchers then ‘rewired’ the gene expression so that it was fine-tuned to produce a controlled release of insulin to a type 1 diabetic male mouse model1. Specially engineered cells were implanted and stimulated using World Health Organization (WHO)-approved and US Food and Drug Administration (FDA)-licensed acupuncture needle electrodes. The stimulation occurred once per day at 4.5 V for 10 seconds, and it was found that enough insulin was produced to restore the sugar levels of the mouse to normal levels.
Fig. 2: The DART System's Adaptation and Validation for Treating Type 1 Diabetic Mice
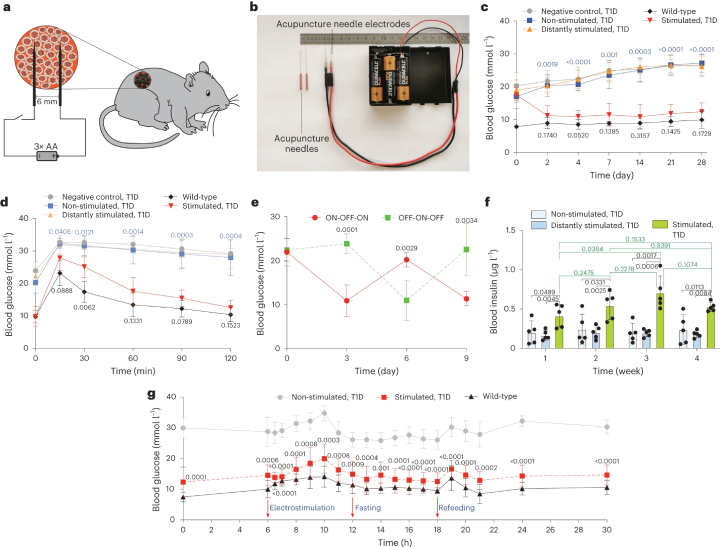
a. A diagram illustrates how DART-engineered cells, encapsulated and implanted in mice, are stimulated. b. An image displays customised acupuncture needles connected to batteries. c. Fasting glycemia was monitored over four weeks in three groups of type 1 diabetic (T1D) mice with DCINS cell implants, including non-stimulated, stimulated at the implantation site, and distantly stimulated mice. Controls included wild-type and T1D mice without implants. d. Intraperitoneal GTT was conducted three days post-implantation after an 8-hour fast. e. The reversibility of DART-controlled glycemia was tested, with ON-OFF/OFF-ON stimulation reversal every third day at 4.5 V DC for 10 seconds. f. Blood insulin levels were profiled weekly for a month, with both stimulated and distantly stimulated groups treated with 4.5 V DC for 10 seconds daily. g. Blood glucose fluctuations were analysed on day 4 post-implantation, with samples collected at various times for glucose analysis in wild-type and T1D mice, both non-stimulated and stimulated with DC 4.5 V for 10 seconds. All data are presented as mean ± standard deviation, with n = 5, and values normalised to the wild-type group. Significance in mean value differences is indicated. (Click to enlarge)
Conclusion
This technology has the potential to enable wearable electronic devices to directly program therapeutic and metabolic interventions. While the DART system was used to control insulin in this study, it was a proof-of-concept study to show that the system worked, and it’s believed that it could be adapted to control the in-situ production and dosing of a wide range of biopharmaceuticals, gene therapies and cell-based therapies.
References
1. Huang J. et al., An electrogenetic interface to program mammalian gene expression by direct current, Nature Metabolism, (2023).
2. Versatile bioelectronic interface (VIBE): Real-time profiling of bio-analytes and hormone sensing, Research Collection ETH Zurich, (2023).

