Soft Neural Probes: Precision Liquid Metal Printing on the Cranium
15-04-2024 | By Liam Critchley

Key Things to Know:
- Complex Neural Dynamics: The brain's intricate network of neurons is essential for all bodily functions, controlling everything from motor skills to memory and consciousness.
- Impact of Material Mismatch: Traditional neural probes made from rigid materials like silicon can cause a mechanical mismatch at the brain interface, leading to potential inflammation and displacement issues.
- Advancements in Neural Probes: Recent innovations utilize soft, flexible materials like eutectic gallium-indium alloy (EGaIn), which closely match the mechanical properties of brain tissue, reducing adverse reactions and improving probe stability.
- Customizable and Conformable Systems: Cutting-edge techniques involve printing neural interface components directly onto the cranium, allowing for highly customized and biocompatible systems that enhance the accuracy and longevity of neural monitoring.
The brain is the most complex organ in the body. It is a 3D complex network of neurons that continuously generate and transmit signals to communicate within itself and the rest of the body. The brain's neuronal activity is responsible for all functions in the body and, on a macro level, controls the body's function, consciousness, and memory formation.
The complexity of the brain means that any small anomaly within the neurons can have large and drastic consequences, including leading to neurological disorders such as epilepsy, Parkinson’s disease, Alzheimer’s disease, depression, and chronic pain. Neural probes are often used to check if someone is at risk of these disorders, as well as to monitor the activity and progression of any disorders once diagnosed. These are implantable electronic devices that transduce neural ionic signals to electronic signals, allowing the neuronal activities within different regions of the brain to be mapped.
Challenges of Traditional Neural Probe Materials
However, one of the challenges of many neural probes today is that they are based on solid materials— such as metals or silicon. These materials have elastic moduli in the region of hundreds of GPa, which is around seven orders of magnitude higher than the elastic modulus of the brain. This leads to a mechanical mismatch at the probe-brain interface that often causes inflammatory responses from the brain tissue after implantation. These inflammatory responses often cause the implant to move from its intended location, so the required data is not always captured, and the patient can experience discomfort as well.
The use of eutectic gallium-indium (EGaIn) in neural probes, as discussed in recent studies, addresses the crucial issue of mechanical mismatch by offering a soft, flexible material that minimizes tissue irritation and inflammatory responses. This innovation is paving the way for safer and more comfortable neural monitoring systems.
Using soft electronics helps to reduce the mechanical mismatch and the inflammatory responses. A number of studies have shown that soft and conformable neural probes can be created that have a better mechanical match with the brain tissue to reduce inflammatory responses. However, despite the material advances in the probes themselves, they are still operated by bulky and rigid electronics that are mounted within the body. These bulky systems can cause the device to degrade quickly in the body and reduce the effectiveness of the soft neural probes over time. To fully realise the potential of soft and biocompatible neural probes, they need to be accompanied by electronic components and a platform that is also soft, conformable, and biocompatible in nature.
To provide a clearer understanding of the technological advancements in neural probes, Figure 1 from the referenced study vividly illustrates the high-resolution printing process of liquid metal used to create soft, flexible neural probes. This figure not only showcases the intricate details of the probe designs but also emphasizes the precision and adaptability of this fabrication method, which are crucial for ensuring the probes' compatibility and functionality within the brain's delicate environment.
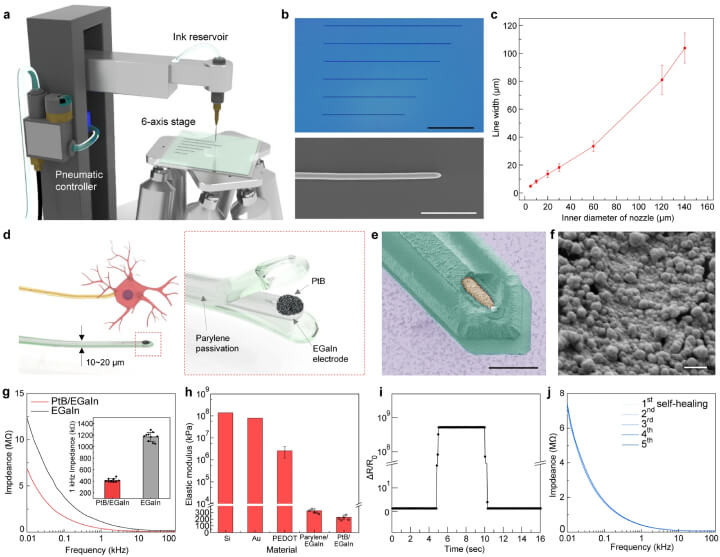
a Schematic of the liquid metal printing system. b SEM images of liquid metal patterns with scale bars at 500 µm (black) and 50 µm (white). c Graph depicting line-widths versus nozzle inner diameter, with error bars indicating standard deviation. d Schematic showing the resemblance between the soft neural probe and neuron. Inset: exploded view schematic. e SEM image of the probe tip colored for parylene (green) and PtB (yellow), scale bar at 10 µm. f SEM of PtB on the probe tip, scale bar at 500 nm. g Impedance spectroscopy for EGaIn with and without PtB coating; inset shows impedance at 1 kHz. h Elastic moduli comparison of materials like silicon, gold, PEDOT, and EGaIn coatings, with standard deviation errors. i Real-time resistance monitoring of PtB/EGaIn during disconnection and reconnection. j Impedance of PtB/EGaIn through cycles of disconnection and reconnection.
Incorporating advanced materials like liquid metals into the electronics of neural interfaces offers a revolutionary approach to ensuring the entire system remains soft and biocompatible. This technique, highlighted in recent research, optimizes the functional longevity and effectiveness of implanted neural probes.
Looking at the Compatibility of the Whole Neural Interface System
When looking at the biocompatibility of the entire implant system used to build the neural interface, there are many subsidiary electronic components that are used for collecting the raw data signals from the neural probes and transmitting them from the implant to an external server in the hospital ready for analysis. Additionally, the flat and rigid shape of printed circuit boards that connect the probes to the subsidiary electronics also restricts the long-term use of neural probes and deteriorates over time due to the mechanical mismatch with the surrounding tissue.
Stretchable electronics are seen as the way forward as they can mimic the natural curvature of the body and not induce any mechanical mismatch that promotes inflammatory responses. However, another key aspect is that the electronics—especially the circuit board—need to be tailored to the individual patient, as it is the circuit board that has the largest interface with the surrounding tissue and has the biggest bearing on the mechanical mismatch beyond the probes themselves. The electronic systems need to be tailored to the patient because each person’s cranium and brain are different sizes, and the location of the neural probes is going to be different depending on which region of the brain is being analysed. So, there’s a call to develop approaches that can meet this level of customisable design depending on the patient and the procedure.
Customizing neural interfaces through direct printing on the cranium ensures each system is uniquely tailored to the patient’s anatomy, enhancing both the efficacy and comfort of the treatment. This method, which utilizes precise placement and materials like liquid metals, can significantly improve patient outcomes by reducing the risk of mechanical mismatch and subsequent tissue response.
New Neural Interface System That Offers Multi-Component Flexibility
Researchers have now taken to printing the neural probes, circuit boards, and subsidiary electronics directly onto the brain and cranium in an effort to meet the customisability and soft electronic demands. The result is a soft and conformable neural interface system that can monitor the single-unit activities of neurons with long-term stability. The components of the neural interface system were printed using a eutectic gallium-indium alloy (EGaIn; 75.5% gallium, 24.5% indium by weight), which is a gallium-based liquid metal.
The liquid metal soft neural probes that were printed had a subcellular-scale diameter, with adaptable lengths, and they were implanted in the brain. Liquid metal circuits, interconnects, and subsidiary electronics were all directly printed onto the surface of the cranium, enabling the complete system to have a conformal integration to the body and better biocompatibility. The circuit system printed onto the cranium was also able to transmit the measured neural signal wirelessly to a smartphone. The signals of the neural probes were also enhanced by the formation of platinum nanoclusters on the neural probes at the neuron-probe interface.
The subcellular scale of the neuronal probes printed were structurally and mechanically similar to neurons. The lengths of the probes could be controlled by the printing of the liquid metal through a capillary nozzle, ensuring that the probes could be precisely modified to various depths and regions of the brain depending on the procedure and regions of interest. The soft modulus of the printed neuron probes was able to restore their electrical conduction properties under extreme deformation due to the self-healing capabilities of the liquid metal.
The application of liquid metal in neural probes not only allows for adjustable lengths and subcellular-scale diameters but also introduces a significant improvement in the electrical conductivity and mechanical properties of the probes. This adaptability ensures that the probes can be finely adjusted to target specific areas of the brain, enhancing the precision of neural monitoring and treatment strategies.
Advancements in Neural Interface Applications
The researchers also demonstrated the ability to form miniaturised and conformal circuits on the cranium of a live mouse and took wireless neural recordings of the brain activity. The probes were used for neural recording and behavioural testing on freely moving mice. The local field potentials (LFPs) and single unit spikes across different regions of the brain were investigated over a long recording time of 33 weeks—showcasing the adaptability of the process to create printable neural interfaces that can be used to measure different regions of the brain across long time periods without degradation.
The recording of the neural signals was performed using both Wi-Fi and a near-field communication (NFC) system. Embedding the NFC communication system into the scalp achieved positive results and the transfer of data for analysis. On the other hand, the Wi-Fi approach was not as successful because the components were not as integrative into the body as the NFC approach. This is because the printed Wi-Fi-enabled platform was larger and had bulky battery requirements.
There’s been a lot of success in creating complete printable neural interface systems directly onto the cranium. The challenges with the Wi-Fi platform open the possibility of further research in the area towards creating more efficient printable batteries using biofluids as the electrolyte. The ability to create both soft neural interfaces and conformal circuity on soft biological tissues could also help to further both the neuroscience and bioelectronic fields in the years to come.
Reference:

