Changing lithium ion cathode expected to improve charging times
28-05-2019 | By Rob Coppinger
Consumer electronics, solar grid storage and electric vehicles could all see improved battery performance for charging if vanadium disulfide is added to a battery’s lithium cathode and cobalt oxide removed.
A battery consists of two electrodes, an anode and a cathode, and an electrolyte. A battery charges and discharges when ions move between the two electrodes, and a lithium ion battery is no different. Researchers at Rensselaer Polytechnic Institute have found a way of creating a lithium ion battery that can charge in five to ten minutes and still retain its full capacity. Most batteries need 30-60 minutes. In a typical lithium-ion battery, the anode is graphite and the cathode is lithium cobalt oxide. The Rensselaer Polytechnic Institute solution is to alter the cathode by replacing the cobalt dioxide with vanadium disulfide.
“There will come a day when there is not enough cobalt on the planet,” said Rensselaer Polytechnic Institute professor of mechanical, aerospace, and nuclear engineering, Nikhil Koratkar. “It [vanadium disulfide] gives you higher energy density, because it’s light. And it gives you faster charging capability, because it’s highly conductive. From those points of view, we were attracted to this material.” He adds that lithium cobalt oxide is not very conductive and that is what has limited the charging speed.
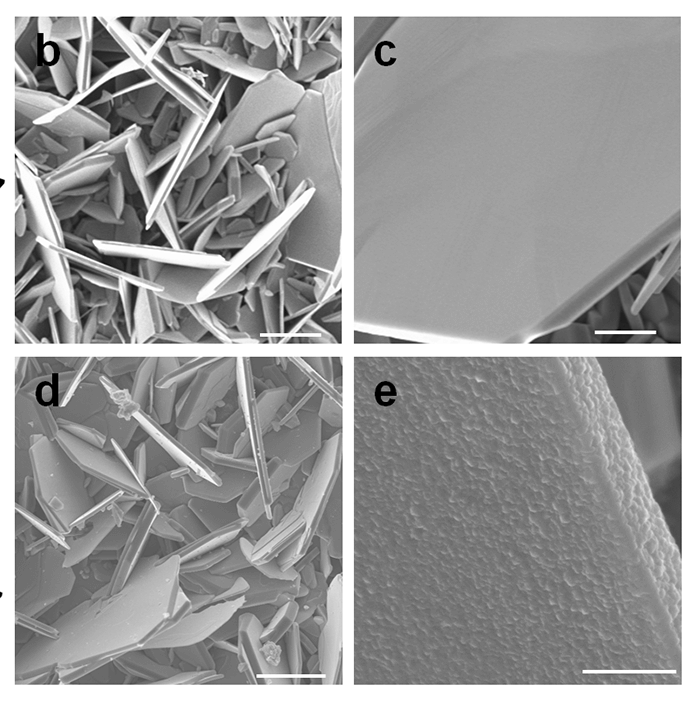
Vanadium disulfide flakes with and without a titanium disulfide coating. Credit: Rensselaer Polytechnic Institute
The experiments Koratkar’s team has carried out to date have involved batteries that are small, a type of battery called a coin cell; because they are the size of a coin. “We want to demonstrate the concept in larger form factors, pouch cells. They’re used in cell phones and laptops. If we get the funding, we hope to do it in the next one to two years,” explained Koratkar.
Stability
Koratkar explained that previously researchers had been challenged by vanadium disulfide’s instability. This instability would see battery life shortened. Koratkar’s Rensselaer researchers found out why vanadium disulfide is unstable and how to stop that and make it stable. The instability is an asymmetry between the vanadium atoms’ spacing, which is caused when adding lithium. The solution is to coat the vanadium disulfide with a nanolayer of titanium disulphide. This stopped the degradation. The titanium disulfide acts as a buffer and gives mechanical support.
With that instability obstacle removed, the researchers found that the coated vanadium disulfide electrode could have a high specific capacity. Due to vanadium and sulphur’s small size and weight they can provide a higher capacity and energy density than cobalt oxide. This could lead to a compact battery design.
Koratkar added that cobalt is only mined in Africa and in the future there maybe supply issues and so alternatives need to be found. Lithium iron sulphate is another alternative that is being investigated by battery researchers. Koratkat sees vanadium disulfide as lighter because it does not contain iron and he said it is environmentally benign.
